In early January, a federal agency alert made clear what many already knew: that crude oil from the Bakken formation is more prone to explosion than other types of crude oil. The warning came after tank cars carrying Bakken oil exploded in three separate railroad accidents in Alabama, North Dakota, and Quebec. It’s a worrisome finding for the hundreds of communities that host loaded oil trains every week.
Let’s take a closer look at some particular issues with Bakken oil.
What’s different about Bakken oil?
Bakken oil is a type of “light sweet crude,” a relatively high quality oil that is easier to refine into commercial products, but also easier to ignite. A few decades ago, light-sweet crude was the dominant oil type in the US. Light oil is by no means new to the industry, but the recent boom in oil extraction in the Bakken and similar deposits elsewhere does represent a new and unexpected development for the industry.
What’s more, the large-scale rail transport of crude oil is a very recent phenomenon. From 2009 to 2012, for example, oil-by-rail shipments grew from fewer than 11,000 railcars nationally to well over 230,000. That astronomical growth has continued in 2013 and early 2014.
Is Bakken oil more flammable than crude oil from other sources?
Yes. This was confirmed by a the US Pipeline Hazardous Material Safety Administration (PHMSA) in a January 2 safety alert to the general public, emergency responders, and shippers and carriers of oil. PHMSA warns:
…recent derailments and resulting fires indicate that the type of crude oil being transported from the Bakken region may be more flammable than traditional heavy crude oil. Based on preliminary inspections conducted after recent derailments in North Dakota, Alabama, and Lac-Megantic, Quebec involving Bakken crude oil, PHMSA is reinforcing the requirement to properly test, characterize, classify, and where appropriate sufficiently degasify hazardous materials prior to and during transportation.
The PHMSA findings were corroborated by the industry-oriented Bakken Shale blog, calling it “flammable like gasoline.” The “flash point”—the lowest temperature at which ignition can occur—is lower for Bakken oil than for lower grade crude oils, which means that Bakken crude is particularly flammable. The post also warns that when flammable gases are dissolved in oil, the oil should be “degasified” before transportation.
What are these more flammable components in Bakken oil?
DeSmog Blog explains what is likely behind the explosions: Bakken oil contains volatile organic compounds (VOCs). As one piece of evidence, consider a permit application by Marquis Missouri Terminal LLC, the company that was to receive the oil from the BNSF train that exploded in North Dakota. In its application to construct a crude oil storage and receiving facility, Marquis reported that the oil was expected to contain Toluene, Xylene, Benzene, and Hexane—all VOCs. The estimated maximum weight of each component in the oil was 5 percent, 5 percent, 2 percent, and 3 percent, respectively, for a maximum total of 15 percent of the oil by weight.
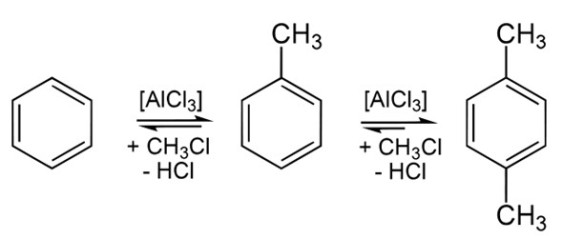
Laboratory reaction scheme showing conversion of Benzene (left) successively to Toluene, then Xylene (right). Xylene also exists in other Isomers, with the methyl (CH3) groups attached to the benzene ring in different positions relative to each other. (Source: Wikimedia Commons)
A video conversation with actor Mark Ruffalo, founder of the organization Water Defense, and the group’s chief scientist Scott Smith, elaborates on the risks of Bakken oil. Smith obtained samples of Bakken oil in which he detected Benzene, Toluene, and Xylene. (His results are posted online by DeSmog Blog, here.)
Others have hypothesized that the culprit is hydrogen sulfide, a corrosive and extremely hazardous gas, that is present in high concentrations in some stocks of Bakken oil.
Why does Bakken crude contain more flammable components?
Scott Smith, the Water Defense chief scientist, explains the role played by the peculiar geology of the Bakken formation. Oil there is found “trapped” between layers of shale rock about 2 miles below ground with no surface outcropping that might allow volatile or gaseous compounds to escape. As a consequence, when the oil is extracted it often contains high levels of VOCs or other hazardous compounds. In fact, Smith estimates that up to 40 percent of the material in a tank car carrying Bakken oil may be volatile organic compounds, and therefore more flammable than the oil itself.
The North Dakota Department of Health has confirmed that Bakken oil contains more VOCs than initially expected, but federal PHMSA sampling data has not yet been released.
John Abbotts is a former Research Consultant to Sightline who occasionally submits material that Sightline staff turn into blog posts.
Update March 7, 2014: A newly released federal investigation found, among other things, that:
The flash point obtained for the occurrence crude oil samples was significantly less than 23 °C…
The occurrence crude oil’s properties were consistent with those of a light sweet crude oil, with volatility comparable to that of a condensate or gasoline product.
The occurrence crude oil samples were taken at atmospheric pressure. This could lead to an underestimation of the crude oil’s volatility due to evaporation loss of very light constituents.
The large quantities of spilled crude oil, the rapid rate of release, and the oil’s high volatility and low viscosity were likely the major contributors to the large post-derailment fireball and pool fire.
The occurrence crude oil contained concentrations of BTEX that were comparable to typical values reported for crude oils. This explains why concentrations of benzene and other VOCs well above exposure limits were detected at the derailment site.












Curious
When you graph the number of accidents per year against oil-filled-railcar miles traveled, over the last 20 years or so, what does the line look like?
Eric de Place
Interesting question. I don’t know the answer, but on some level it may not matter too much. The increase in oil railcars is so incredibly dramatic that even if the rate is declining the absolute number is increasing. See here for more: http://www.sightline.org/2013/11/14/us-oil-train-trends-four-basic-pictures/
And of course what really matter is how often these things could explode!
Chris Troth
Yeah. I’m not at all mollified by the fact that only a small percentage of these trains have so far and, presumably, will in the future derail and explode. The fact is that the number of rail transport trips is increasing rapidly with even further increases planned. Unless the rate is zero (unrealistic), there will be more derailments, more explosions, more contamination and more deaths, inevitably.
I expect that the fact that the accident was unlikely is cold comfort to the victims and survivors of the disaster in Lac-Megantic.
There are a lot of risks that accompany life in the modern world, some are less addressable that others. Exploding oil trains from the Bakken is one that we can, should and need to contain. As will all of our fossil fuel dependency, we have been far too complacent already.
John Abbotts
Hello Chris,
Thank you for your comment. If you have taken a look at the video conversation linked above (repeated here, at https://www.youtube.com/watch?v=QUEieToUeNg
you will hear Scott Smith talk about two basic causes for explosions: old oil tanker cars that are poorly designed to withstand accidents; and the high levels of volatile chemicals in Bakken oil. In fact, he discusses evidence that the North Dakota train tank cars may have exploded first, causing the derailment, rather than the converse.
In addition, as comments on some Sightline posts have mentioned, the old oil tank cars, labeled DOT-111, have been allowed to stay in service, despite their poor resistance to pressures and impacts during accidents. Sightline may have more on DOT-111s in the future, but as Scott Smith noted, federal agencies and lawmakers have been pressured by moneyed interests (FDR called them “malefactors of great wealth,” a phrase akin to the more recent “truly greedy 1 percent”) to allow these tank cars to operate without tighter regulation.
One last observation, and I should make clear that I speak for myself, not necessarily Sightline: You may have seen in the news calls for more corporate social responsibility at Davos, Switzerland, where the economic elite meet annually, link at http://www.cnbc.com/id/45856248
Of course, with regard to your last sentence, a little corporate responsibility could go a long way in building a more sustainable energy future. But if energy policy continues to be bought by the Koch brothers and their ilk (see “malefactors” above), then it is up to ordinary citizens to organize and press their governments to regulate not only exploding oil trains, but energy policy as well (and economic policy, etc.).
That’s it for my soapbox, at least for tonight. Thanks again for reading and commenting. I always appreciate feedback, positive or negative, on our posts. It tells us that people are reading, and whether what we write comes through clearly.
John Abbotts
Hello Curious,
Thanks again for you question,
Not to belabor the obvious, but Sightline’s Headlines section today contains a link to a NYT article, titled “Accidents Surge as Oil Industry takes the Train, link at http://www.nytimes.com/2014/01/26/business/energy-environment/accidents-surge-as-oil-industry-takes-the-train.html?_r=0
The article mentions the accidents in Quebec, Alabama, and North Dakota, and reports that a 100-car oil train can take five minutes to pass a specific point, which is generally consistent with Eric’s estimates in the Methodology post on another Sightline series, link at http://www.sightline.org/2013/09/12/the-methodology-behind-the-wrong-side-of-the-tracks-series/
The NYT article also contains the following paragraph, which provides some numbers:
“While the safety record of railroads has improved in recent years, the surge in oil transportation has meant a spike in spill rates. From 1975 to 2012, federal records show, railroads spilled 800,000 gallons of crude oil. Last year alone, they spilled more than 1.15 million gallons, according to the Pipeline and Hazardous Materials Safety Administration. And that figure does not include the Casselton spill, estimated at about 400,000 gallons.”
Other items in the article include a link to another NYT article describing how U.S. and Canadian regulators are trying to tighten standards, and the controversy with old tanker rail car design.
The article also includes a map of rail lines, which is skewed toward the Midwest U.S. But if one starts on that map from the Bakken formation (the dark area in ND and Montana), BNSF trains are already taking Bakken oil west across the top of Montana, then through Northern Idaho, Spokane, and a similar route along the Columbia to that described in our Wrong Side of the Tracks posts. Not to puff up Sightline, but the post showing examples of grade crossings in Spokane County, with a link to the entire series, is at
http://www.sightline.org/2013/09/19/how-coal-and-oil-trains-will-block-traffic-in-spokane/
Well, okay, it does puff up Sightline, but the shameless self-puffery is my responsibility alone; and after all, Sightline did do the work already, and it has been covered in the media.
Thanks again for your question. I this may provide useful information, and I hope Sightline’s editors will tolerate my long-winded answer.
kim
I would also like to know how many barrels/gallons/whatever comparable volume have been leaked by rail as opposed to leaked by pipelines. There is a push right now (12/2014) to build a pipeline to Illinois, through Iowa. The mantra is that pipelines are the safest transport medium but, when asked at a public informational meeting, the “energy company” pushing to build this pipeline told us that the only critera for “safest” was the number of human injuries or lives taken.
I’m pretty sure that pipeline leaks in the USA in the past 14 years far exceed pollution volume, benzene and other VOCs in our soil and into our water, etc. etc., than railcar accidents.
My preference is to deny transport to the Bakken product, or to simply continue with rail —- at least we can detect rail crashes, while they cannot quickly or easily tell if a pipeline is pumping hundreds of thousands of gallons of petroleum and VOCs into the ground.
Ryan Ferris
Who has described the difference in TRI (Toxic Release Inventory) between Bakken or other shale oil refining and “conventional” oil refining?
John Abbotts
Hello Ryan,
I should note first that I am not an expert on the TRI Inventory, but a bit of noodling on TRI web pages tells me that TRI may not have the information to answer your question.
That’s my executive summary, and the details of my noodling follow:
I started with the TRI 2013 National Analysis by Industry Sector, link at http://www2.epa.gov/sites/production/files/2015-01/documents/4-tri-na-industry-sectors.pdf
In that document, information on Petroleum Refining begins on pdf p. 15, which notes that 151 facilities in that sector report to TRI their waste management activities, including emissions (“disposed or otherwise released”) data to TRI.
pdf p. 19 of the same document reports the top three chemicals released: nitrate compounds, ammonia, and sulfuric acid.
The Industry Sector Analysis also had a direct link to one refinery, at http://oaspub.epa.gov/enviro/P2_EF_Query.p2_report?FacilityId=8263WNTLPR27HIG&ChemicalId=000071432&ReportingYear=2013&DocCtrlNum=
That page displays information for one refinery on Benzene releases, which is one volatile organic chemical; other chemicals can also be selected with a drop-down list on the page. The information for a given chemical may be displayed by waste management category, including releases; production index; and releases normalized to production, among other options.
So at this point, my conclusions on TRI are that one can find release data on individual chemicals at individual refining facilities, but release data are aggregate over one year. To answer your question, a facility would have to report its chemical emissions by the sources of the oil that it refined at different times during the year; and emissions would have to be normalized to production to provide a common comparison point.
My understanding of TRI is that it would be very difficult to get information that specific from refineries, and they would probably regard it a trade secret. Moreover, environmental laws do allow companies to claim specific data as Confidential Business Information, not to be released to the public.
So, my regrets Ryan. It may be possible to obtain the information you seek, but it would probably have to come voluntarily from individual refineries. The above material summarizes what I have been able to find on TRI in a short time, and you are certainly welcome to conduct your own data mining from the web pages, but for now, my answer to your question is:
I do not know who has described the information you want.
Thanks for your interest in Sightline and your question; my regrets that providing an answer may need its own research team.
Allan Freedman
Which is more flammable or has a lower flash-point; Bakken oil or automobile-grade gasoline?
John Abbotts
Hello Allan,
Thank you for your question. According to Wikipedia, gasoline has a flashpoint of -43 degrees C; link at http://en.wikipedia.org/wiki/Flash_point
I found a Material Safety Data Sheet (MSDS) for Bakken crude issued by Conoco Phillips, reporting on page 3 that Bakken is “extremely flammable,” with a flash point less than -29 degrees C. So gasoline may have a lower flash point, but both substances are highly flammable. You may have to zoom in on the page to see details more clearly, but the link to the MSDS is at
http://walpage.com/read/_px.jjj.pbabpbcuvyyvcf.pbz_ds.sustainable-development_ds.Documents_ds.2014.05.30_ol.825378_ol.Bakken_ol.Crude_ol.Oil,_ol.Sweet.pdf.html
Irwin Mainway
The Northwest suburbs of Chicago had a coal train derail and pile up – 27 coal cars on an overpass/embankment, collapsing it. Extreme heat effecting the rails was to blame.
Suppose a similar derailment involved Bakken crude oil tankers occurred in Chicago or another metropolis?
A gargantuan inferno just upwind of millions of people. How would it be put out? Just letting it burn is not an option.
Edward David Perrotti
why not separate that volatile component before shipping it….
Eric de Place
Because it doesn’t work. In fact, the oil train that blew up on May 6 was treated for volatility: http://trib.com/business/energy/oil-company-shipment-in-north-dakota-train-derailment-had-been/article_072d1cf4-b5a2-5051-b435-bfc92faa5a8f.html
(It was also traveling slowly: just 24 mph.)
Moreover, trains carrying heavy Canadian crude have also exploded on several occasions. So there is good reason to believe that the current methods of treating the volatile components of Bakken oil are missing the point.