In the stormwater world, if a rain garden is releasing more pollution into the environment than it’s capturing, word gets around.
So when the city of Redmond crunched its first flush of data from a new roadside rain garden and discovered the water coming out of it was tainted with alarming levels of phosphorus, nitrates, and copper, the stormwater community took notice. Washington State regulators went on the record to say that they would be studying the data and possibly revising their rain garden recommendations. Proponents of the technology fear that the results will be overblown and exploited by skeptics of so-called low-impact development solutions.
But even city officials in Redmond caution that they’re far from giving up on rain gardens.
“It definitely has not lost its merit in my mind,” said Andy Rheaume, Redmond’s senior watershed planner.
Indeed, there’s a decade worth of data showing that rain gardens and related “natural” technologies are effective at treating polluted stormwater runoff. They can do a terrific job soaking up the renegade rain water, diverting it from house basements and preventing it from scouring streams or causing overflows of sewage. And numerous studies demonstrate that rain gardens will filter out and capture a toxic mix of heavy metals, petroleum pollutants, particles and nutrients. In fact, the Redmond rain garden did treat some of the pollution gushing into it.
But rain gardens aren’t fool proof. Depending on the design of the system and the soil mix that’s used, a rain garden’s ability to remove pollutants can vary—and vary dramatically.
So what is a city or county stormwater engineer to do? Don’t panic.
“We’ve been promoting the message ‘Don’t throw away the baby with the bathwater,’ ” Rheaume said. “We’re pretty sure that (low-impact development) is here to stay.”
New Street, New Rain Gardens
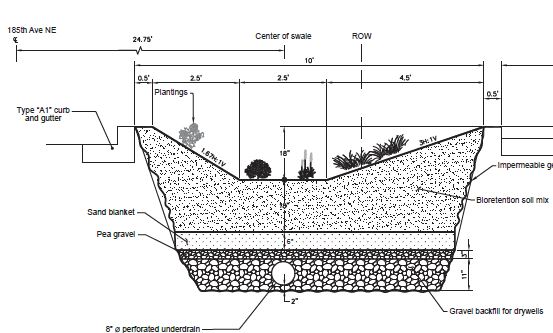
In fall 2011, Redmond took advantage of a road-building project to construct some green stormwater infrastructure at the same time. The city installed pervious concrete sidewalks and three rain gardens that stretched a combined 410 feet along 185th Avenue NE. The rain gardens, or “bioretention systems,” collect and absorb water from about one-quarter of an acre, including arterial roads that are surrounded by industrial use.
Because the rain gardens were situated above an aquifer that provides the city’s drinking water, engineers lined the cells of the gardens with an impervious geomembrane to stop the runoff from seeping into the groundwater.
The bioretention systems were built according to Washington standards with 18 to 36 inches of “bioretention soil mix” that was 40 percent compost and 60 percent sand. The soil was planted with grasses and small shrubs. Beneath the soil layer was 6 to 8 inches of sand, 3 inches of pea gravel, and 11 inches of gravel backfill in the lowest layer (see diagram in Figure 4).
In the gravel layer, the engineers installed 8-inch underdrains that were designed to carry overflow stormwater out of the rain gardens and into nearby Bear Creek.
Given that the city depends on groundwater for drinking, officials wanted to know if the stormwater carried contaminants that could pose any risk to residents should it percolate into the aquifer. Stormwater regulations advise that engineers not concentrate stormwater runoff near stores of drinking water. But in truth there’s not a lot of data in the Northwest on what’s seeping out of rain gardens. To help answer this question, Redmond officials received a grant from the Ecology Department for $500,000, which covered 75 percent of the monitoring project; Redmond paid the remaining $165,000.
So three months after construction wrapped up, from February to July 2012, researchers monitored one of the cells. They grabbed stormwater samples where it entered the rain garden and as the “effluent” flowed out of it.
By taking the samples so soon after construction, researchers knew they were collecting some of the first flushes of water that would likely have unusually high levels of nutrients from the soil’s compost. But even with these expectations, the results –– at least for some of the chemicals –– were worrisome.
The Trouble with Compost
Scientists have known for years that rain gardens can be finicky.
In Michael Dietz’s 2007 review of low-impact development strategies, he reported widely varied performances from rain gardens tested in Maryland, New Hampshire, Connecticut, and North Carolina. All removed metals including copper, lead, and zinc. But while most rain gardens effectively captured phosphorus and nitrogen-containing pollutants, in a couple of cases they released more of these pollutants in the effluent than they trapped.
Then in 2012, the East Coast’s rain garden rock stars William Hunt, Allen Davis, and Robert Traver teamed up to deconstruct bioretention systems. Their publication gave point-by-point advice on how to tweak rain gardens depending on which pollutants you’re most concerned about removing.
Want to clean petroleum pollutants out of your water? Don’t forget to spread a couple of inches of mulch on your garden, the scientists advised. If nutrients such as phosphorus, nitrogen, and nitrates are your targets, dial back the amount of organic material, including compost.
The soil used in a rain garden has a big effect on what seeps out of it, experts say.
“We and others have known this for years and continue to work on media,” wrote Davis, a professor in the University of Maryland’s Department of Civil and Environmental Engineering, in an email exchange.
“Compost will leach (phosphorus) and some composts will leach a lot of (phosphorus),” he wrote. “This has been known for many years.”
So here’s the challenge: How do you build a rain garden that contains enough organic material to support plant life, hold enough water through the summer, and create a habitat where pollutant-gobbling microbes will hang out, but not add so much compost that the garden disgorges too many nutrients, potentially fouling waterbodies downstream?
Washington State’s Stormwater Management Manual for Western Washington calls for 35 to 40 percent compost in the soil mix for rain garden systems. Scientists at Washington State University are investigating whether lower amounts of compost can keep plants happy while releasing lower levels of nutrients. They’re also looking at whether compost that’s sat around and aged for a while will shed less phosphorus and nitrogen.
Redmond’s Numbers

Roadside rain garden, Lisa Stiffler
In Redmond, automated devices collected runoff during storm events, resulting in eight separate samples for nitrogen, phosphorus, copper, and other pollutants.
Nitrogen in the form of nitrate and nitrite is particularly worrisome because high levels of these chemicals in drinking water can cause “blue baby syndrome” in which the nitrate binds to hemoglobin in the blood, robbing oxygen from the baby’s cells. Phosphorus is a problem primarily when dumped in rivers, lakes, and enclosed sea water where it can trigger harmful blooms of algae. And copper is known to cause problems for salmon and other aquatic organisms.
The results from Redmond showed dramatic increases in the amount of pollution leaving the garden, at least for certain chemicals:
Average Amount of Pollutants Entering and Leaving Redmond Rain Garden* (mg/L)
By contrast, the garden did a good job of filtering pollutants including fecal coliform, motor oil, and zinc. In those cases, the levels in the effluent leaving the rain garden were lower than in the stormwater that entered it.
Curtis Hinman, who leads the green stormwater research at WSU’s stormwater center in Puyallup, said the data were high by comparison to other studies and research that he’s done.
“We haven’t seen any numbers like that ever, even remotely close,” said Hinman, whose rain garden pollution results have not yet been published.
In the Northwest, the cities of Seattle, Portland, and Tacoma have sampled pollutants flowing out of some of their rain gardens.
In Seattle, scientists tested runoff as it flowed into and exited a rain garden on NW 110th Street in a residential neighborhood in the northwest area of the city (Table 2). The rain garden is composed of 12 individual bioretention cells that allow the water to pour from one to the next, and the water was collected from the first and eleventh cells.
Researchers in Portland sampled stormwater runoff that flowed out of two rain gardens at the Oregon Zoo parking lot. They compared those values, which were collected about six months apart, to untreated runoff sampled at the same parking lot (Table OZ-3).
Officials in Tacoma have started monitoring the water going into and out of bioretention systems at R Street and 44th Street in the Salishan residential development (see “Context” slide in this presentation).
It isn’t fair to do a rigorous apples-to-apples comparison of these rain gardens to the Redmond system, as the size, designs, compost mix, and age of the facilities are different. But even with these limitations, they can still provide some useful trend information.
If you compare the runoff entering and leaving each garden (or in Portland compare the rain garden runoff to the untreated stormwater), you see that the nitrogen and phosphorus values are frequently higher in the water exiting compost-containing rain gardens.
Comparison of Pollution in Runoff Entering and Exiting Northwest Bioretention Facilities**
And again, given caveats big as a stormwater settling pond, you can also compare the concentration of pollutants leaving the green stormwater facilities.
Amount of Pollutants Leaving Northwest Bioretention Facilities (mg/L)
 Despite the risks of making such a comparison between very different systems, again it is clear that the Redmond numbers are often orders of magnitude higher than pollution levels taken from other Northwest rain gardens.
Despite the risks of making such a comparison between very different systems, again it is clear that the Redmond numbers are often orders of magnitude higher than pollution levels taken from other Northwest rain gardens.
Potential Pollution Culprits
So what’s going on in Redmond to create such a spike in the pollution measurements? Could this problem be more widespread, or is this an isolated case?
The first thing to consider is the design of the Redmond bioretention system. The rain gardens on 185th Avenue NE were built with an impervious lining to protect drinking water supplies and an underdrain, so in heavier rainstorms some of the stormwater filters through a few feet of soil and then drains quickly out of the garden.
In rain gardens without this design –– which includes most of the bioretention systems in the Northwest –– the water can soak as deeply as the soil allows and it might travel a long, long way before reaching groundwater or surface water such as a stream or lake.
Researchers have shown the more time the water spends infiltrating, the greater the opportunity for pollutants to filter out and stick to the soil, or get broken down by natural processes. Short cutting that pathway with an underdrain reduces that opportunity.
The second feature to address is the soil mix. To build a rain garden, you need organic material and sand, and that organic matter needs to be readily available, affordable, and –– particularly for public projects –– a source that is not proprietary. Enter the compost.
Washington State has a relatively extensive program of collecting yard and food waste and turning it into compost for public use. There are state standards that compost producers must meet for the concentrations of various pollutants including metals as well as nutrient levels. The regulations were crafted to make sure the soil was safe for gardens and yards –– not so that it would be optimal for rain gardens.
When the material used for the Redmond project was tested, it had 660 mg/kg of total phosphorus; the state rain garden guidelines recommend compost with less than 100 mg/kg of available phosphorus, which is not directly comparable to total phosphorus. The copper concentrations were 61 mg/kg (the state limit is less than 750 mg/kg), and nitrogen information was not available.
Compost producers weren’t “thinking about using this stuff as a stormwater filter. They were thinking about using this for someone’s garden,” Hinman said.
He and some others in the field suspect there was something unusual with the compost used in Redmond, that perhaps the manufacturer added manure or another nutrient-rich additive that wasn’t disclosed.
There “has to be a higher quality, more controlled material for this kind of application,” Hinman said. “The Redmond monitoring project brings up the question of consistency and reliability and our confidence in getting good (soil) media in the ground for bioretention systems.”
Pursuing a Solution
Redmond officials are planning to build another rain garden this summer, and they’re seeking funding to expand their monitoring program to include six installations.
Their engineers will try different soil mixes in some of the rain gardens, including so-called “amendments” to the soil such as biochar and shredded cedar bark to better capture pollutants.
They will also try a design that incorporates a “saturation zone,” or an area of the rain garden where water will pool underground and create an oxygen-free environment favorable to chemical reactions that remove nitrates from the water.
Rheaume is eager to see what the monitoring will show at the new sites, as well as at the original rain garden.
“We want to see if this thing gets better,” he said.
The preliminary results from Redmond prompted the state Department of Ecology to remind stormwater folks in Washington not to use bioretention within 100 feet of a spring or a well used for drinking water, and not to install an underdrain if it leads to a phosphorus-sensitive body of water. In a move that surprised many, the state went a step further to say it’s considering whether to revise these guidelines to recommend against installing underdrains that ultimately empty into any surface water.
Ecology recently issued new regulations intended to increase the use of low-impact development stormwater treatments, including bioretention. Some cities and counties have challenged the rules, and all sides are waiting for the state’s Pollution Control Hearing Board to take up the issue later this year. Some stormwater experts worry that the Redmond data will be ammunition against Ecology’s regulations.
For now, Rheaume and Hinman will keep testing new soil mixes and designs to optimize what they both see as technology that can ultimately help the environment.
“We don’t have the silver bullet to say what the change is” that will yield better results, Rheaume said, “but we’re in pursuit of it.”
Editor’s note: On May 29, 2013, I added a reference to who paid for the monitoring research done in Redmond.
















Kevin Matthews
Wow, this is great coverage of an issue that is really important to all of us who want to understand how to actually reduce the impact of urbanization on critical natural systems.
Once again it highlights that there are lots of ways that unintended impacts can creep into a system, even a system intended to reduce impacts. There’s no short cut to reducing the net overall impact of urbanization – it may be possible, but it ain’t gonna be easy.
So, pushing back the issue over time earlier in the pollution chain, where inappropriate stuff isn’t dumped on the ground needing to be filtered in bioswales, is likely to prove relatively effective and cost-effective over time.
One sub-issue the article doesn’t quite get to, that I always think about regarding these “natural” treatment systems. It relates to this sentence: “All removed metals including copper, lead, and zinc.”
Where do those metals go, when a bioswale removes them from the water stream? What happens with those metals over a period of decades?
Aaron Clark
Great points Kevin. In answer to your question about where the metals go, the short answer is that these metals are actually important micronutrients for the plants, microbes and fungi that live in these systems. The copper, zinc and other metals are used to create enzymes and other compounds that help the plants survive and thrive. In other words they become a part of the plant, the bacteria and the fungi just as they do in natural systems. The metals themselves aren’t inherently harmful, but in unnaturally high concentrations in our water bodies they can do a lot of harm. I hope that helps.
http://en.wikipedia.org/wiki/Micronutrient#Micronutrients_for_plants
Lisa Stiffler
Kevin, thanks for the comments and question! I want to add to what Aaron said by pointing you to this earlier blog post: Are Rain Gardens Mini Toxic Cleanup Sites?
Indeed some of the metals are taken up by the plants in their roots, and a large amount of the metals simply stick to different components of the soil. Research shows that metals including lead, copper, and zinc are concentrated in the top 8 inches of the soil (see Li and Davis 2008).
As I note in the toxic-site blog post, over the period of many years, if a rain garden is capturing really polluted stormwater runoff, it can make sense to remove some of those top inches of soil and replace it. The idea is that it’s better to have these metals trapped in a contained rain garden than it is having them dumped into sensitive streams, lakes, and other waterways that are more expensive and difficult to clean up.
Steve Erickson
it sounds to me like the compost that was used was not very mature. If it was throughly (and properly) composted, the organics should be much more stable and not leach large proportions of N. I suspect that most of these commercial composts are considered “finished” due to economic pressures sooner than this. After, having a flush of readily available N in a garden will probably be considered a benefit by a gardener.
Also, the proposal to add an anaerobic process is a good idea. These systems will transform nitrates into less soluble, more stable forms of N. In nature, they are called wetlands.
Lisa Stiffler
Thanks, Steve. The good news is that Redmond and others are testing different soil media and compost material that has aged longer. The stuff used in Redmond does seem unusual, though experts acknowledge that if you use a lot of compost, some nutrients are going to be released.
jerry parker
Lisa –
What a great example of good investigative reporting! Makes me proud to be a Sightline contributor. Now, if this level of reporting could just spread …..but you have set the marker.
Jerry Parker
Edee Maurer
Jerry Parker, Jr.? Brother of Avonne? I don’t mean to hi-jack the subject but this turned up on my google search for my uncle.
Sorry and thanks
Robert Traver
I have never been called a rain garden rock star before! Seriously.. a well written article, I will use it in my class tonight. Thanks for clarifying that this is design issue. Ours is 12 years old, works great and reduced nutrient. We have no underdrain, the bowl is 18″ deep, have never replaced any mulch, and simply mixed sand and the original soil to make the media.
Rob
Lisa Stiffler
Ha! And I don’t just throw those titles around — my understanding is that you’re well qualified to wear the mantle of rain garden rock star. Thanks for the additional information on the work that you’re doing.
Jim Clarke
See a new emerging technology for removal of contaminants from storm water called the LilyPad. A nanotechnology coated fibrous mesh that is activated by UV light to break down contaminants and render them benign. It is an amazing process and we are looking for field trial opportunities.